Experiment 2 Identification of a Compound Chemical Properties Answers
Experiment 2 - Physical Properties. If a compound is found to dissolve in water the pH of the aqueous solution should be estimated with pH paper or litmus.

Module 2 Chemical Safety Data Summary Using Chegg Com
The maximum mass usually in grams that dissolves in a fixed mass usually 100 g of solvent at a given temperature.

. Physical Properties flashcards from N Pennisis class online or in Brainscapes iPhone or Android app. Ionic compound composition molecular compound composition melting time and electrical conductivity. Refer to Technique 7A and Figure T7a front portion of your.
To prepare read the Introduction and Experimental Procedure Sections. Identification of a Compound. Experiment 2 Identification of a Compound.
Experimental Procedure Part A a. Criterion means how you test to be sure that the glassware is clean. Physical properties of matter include qualities or attributes such as density odor color melting point boiling point and magnetism.
Prelab Spend 5 minutes doing the following activity. Most organic compounds are nonpolar and thus do not mix with polar molecules like water. Identify at least five observations that are indicative of a chemical reaction.
V volume in mL. If the compound is organic a solvent that will dissolve it can usually be found. Chemical Properties Date Lab Sec.
Chemical Properties Post lab questions 1. Make predictions about the identity of various compounds based on. Chemical Properties of Known Compounds Indicate your observations in the reaction matrix.
To determine the melting point of an unknown compound and then use this melting point as an aid in its identification. Experiment 2 Report Sheet Identification of a Compound. Experiment 2 Identification of a Compound Chemical Properties Pre-Lab Hints 1.
The test tubes were arranged in rows of three for every five rows as follows. Waft the air above the surface of the test tube towards your face. List three properties of your lab partner that help you identify this person as your lab partner.
We will work with strong acids and bases in the next experiment Ionic Compounds 3. In this lab you will explore some of the differences in the properties of organic vs. Chemical Properties of Known Compounds Test NaCl aq Na 2 CO 3 aq MgSO 4 aq NH 4 Cl aq H 2 O l Unknown 1 AgNO 3 aq Precipitat e Precipitate None Precipitate None None NaOH aq None None None None None Precipitate HCl aq.
Jones in Before Lab CP2121. The density of a compound is an intrinsic property which means it is a characteristic property of the compound that can be used to help identify it. Look at a person across the lab room.
Identify a chem ical reagent used in this experiment that can be used to distinguish. Identification of a Compound. 113 -115 o C Benzoic acid mp.
122 o C. Row 1-NaCl row 2- Na 2 CO 3 row 3- MgSO 4 row 4- NH 4 Cl and row 5- H 2 OEach row of test tubes. These properties are often useful for identifying different types of substances.
A reaction has occurred only if at least one of the products is insoluble non-ionized or only weakly ionized. The properties of organic compounds are very different from the properties of inorganic compounds that you have been using up to this point. Identification of a Compound.
Chemical properties of matter in contrast describe how matter interacts with other types of matter. Classify each chemical as an ionic compound molecular compound acid base or salt. The physical state of the unknown compound was observed.
The size and volume of a small clean test tube is 75mm and 3 mL. Test NaCl aq NaCO3 aq MgSO aq NHCl aq HO 1 Unknown AgNO3 aq ppt ppt nr ppt nr ppt NaOH. Chart listing the properties of known compounds.
Physical Properties Tests. Refer to Technique 2 in the front portion of your lab manual. Which are physical properties and intensive properties a property independent of sample size of a substance.
Youre going to work with many types of chemicals in this experiment. If a compound is only slightly soluble in water it may be more. Chemical Properties Deck No Date Lab Sec.
If no compounds or ions remain after cancelling those ions appearing on both sides of the total ionic equation then no reaction has occurred. Look at your lab partner. Most of the ionic compounds are considered by chemicals to be salts and many of them are solid in water.
Identification Of A Compound. Answer Laboratory Questions 3 5 and 6. In this experiment fifteen test tubes were analyzed to see if a chemical reaction would take place.
Solubility of a salt in water at various temperatures Laboratory objective. Tomorrow we do Experiment 2. Experimental Procedure Part A2.
In Experiment 2 the chemical properties of a substance are used for its identification. Ionic and covalent properties lab answer key Lab 05 properties of ionic and covalent bonds answer key. Unit 2- Elements Compounds and Mixtures and.
View the full answer. 1 7 Elements Compounds and Mixtures IC 2 8 9 PhysicalChemical PropertiesChanges Notes IC 2 10 Physical and Chemical Properties and Changes Worksheet IC 2 11 Energy Notes IC X 23 12 14 Physical Chemical Changes Lab HWIC 3 15 16 Unit 2 Review HW 4 X Unit 2 Quiz In class on 921. D mV where d density in gmL.
Bryce Augustine Chem 005- 19 February 2021 Pre Lab 2. Identification of a Compound. The unknown compound will be one of the following.
To determine the density of water ethanol and hexane. As you know from Experiment 1 density can be determined by measuring the mass and volume of a sample and dividing. Lab properties of ionic and covalent compounds answer key pdf.
September 14 2016 by C. Experiment 2 Report Sheet Identification of a Compound. Compounds soluble in water are usually soluble in all the aqueous solvents.
This included the state of matter smell using the wafting technique color and structure. -precipitate appearing or disappearing. Read the bottom paragraph as well as the caption for Fig T2b.
Chemical Properties 09282016 Lab Sec. Describe the technique for testing the odor of a chemical. The criterion for clean glassware is no previous residue on the glassware that could affect the outcome of the experiment.
17 Christie Levy A. M mass in g. A small amount of the unknown compound was placed in approximately 50mL of water and stirred to see if it would dissolve.

Module 2 Chemical Safety Data Summary Using Chegg Com
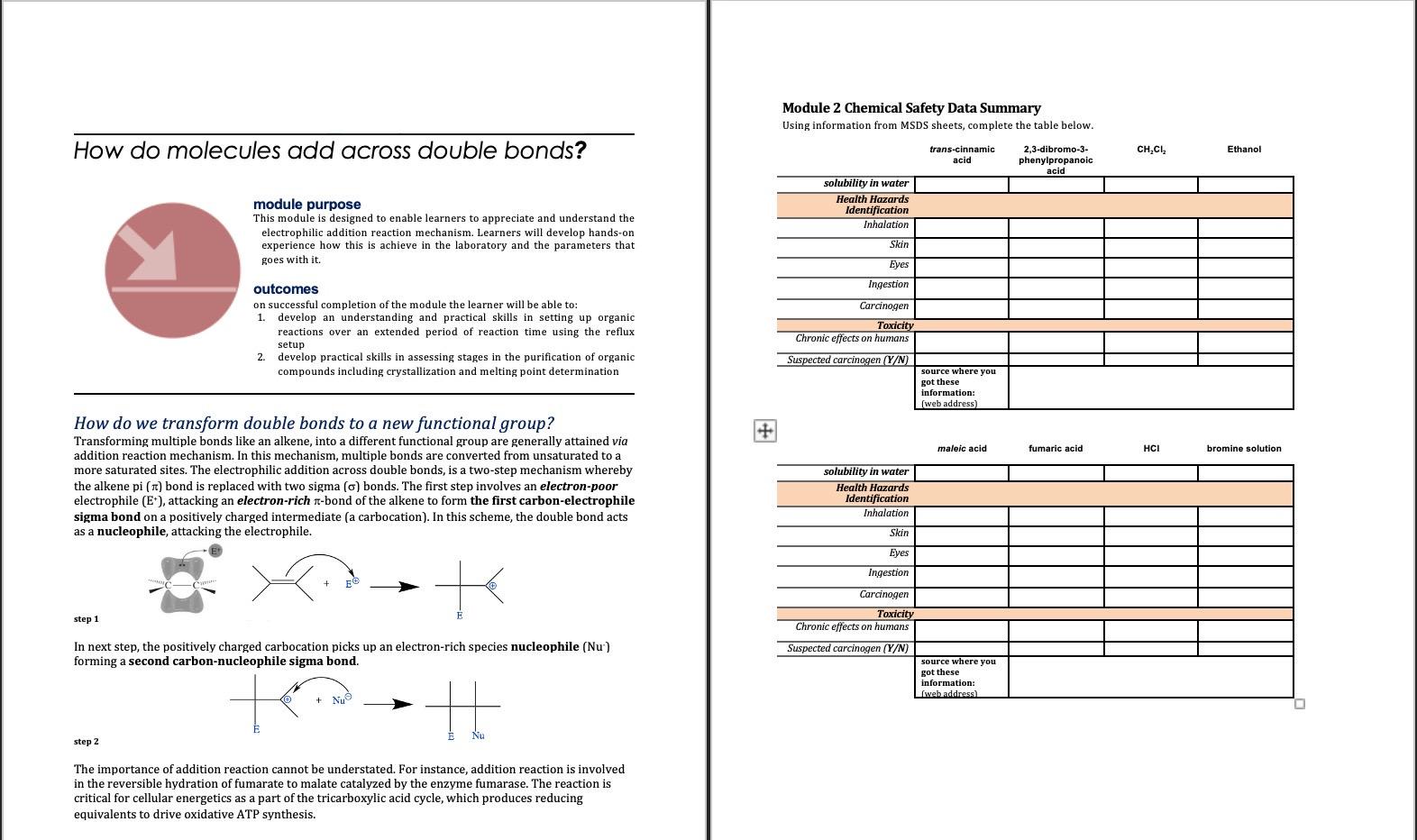
Module 2 Chemical Safety Data Summary Using Chegg Com
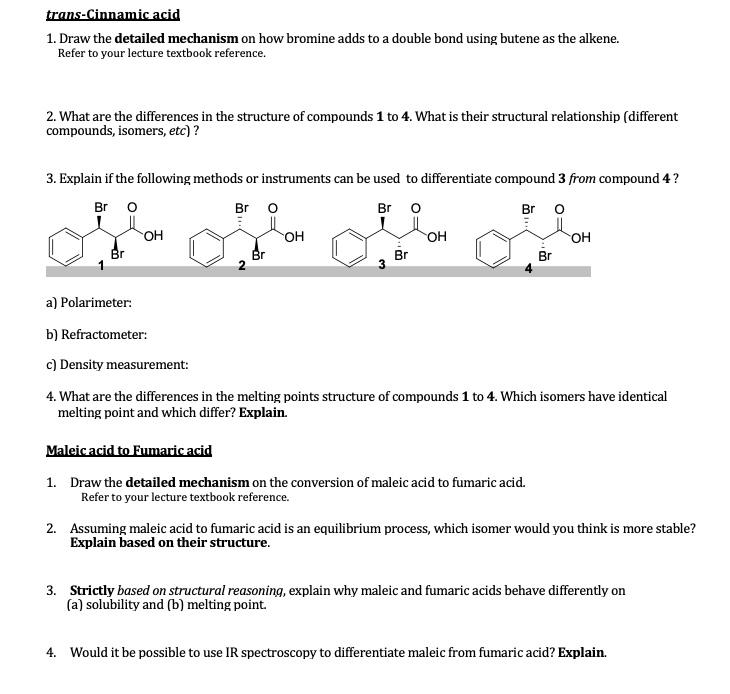
Module 2 Chemical Safety Data Summary Using Chegg Com

Physical Chemical Changes Color By Number Chemical Changes Science Card Sorts Science Task Cards
No comments for "Experiment 2 Identification of a Compound Chemical Properties Answers"
Post a Comment